Aggressive vaccine effort could cut cervical cancer
CHICAGO (Reuters) – An aggressive strategy of vaccinating older women against cervical cancer could deliver a crippling blow against the disease, cutting rates for that type of cancer in half for women through age 45, U.S. researchers said on Saturday.
Using a mathematical model, they showed that vaccinating women in the United States by ages 12 through 45 against the cancer-causing human papillomavirus, or HPV, could reduce cases of cervical cancer by 85 percent for 12-year-olds and up to 55 percent for 45-year-old women.
It could lower rates by 34 to 67 percent for 25-year-old women, Warner Huh of the University of Alabama told a meeting in Washington of the American Society for Microbiology and the Infectious Diseases Society of America.
The model assumed 100 percent vaccination rates, which would be difficult to achieve in the United States.
Most cases of cervical cancer are caused by the sexually transmitted human papilloma virus.
Merck and Co's Gardasil vaccine is designed to protect against HPV types 16 and 18, which are known to cause about 70 percent of all cases of cervical cancer. It also is designed to protect against HPV strains 6 and 11, which cause genital warts.
Gardasil is approved in the United States for use in girls and women ages 9 to 26, but Merck is seeking to expand its use to older women. The thinking has been that girls must be vaccinated before they are sexually active, because HPV is so common.
The vaccine does not protect anyone who has already been infected with one of the strains of HPV.
Huh's calculations included clinical trial data on GlaxoSmithKline's Cervarix vaccine, which is not yet approved for sale in the United States but which is approved in Europe.
He assumed Cervarix gave 95 percent protection against HPV types 16 and 18, and 27 percent efficacy against all other high-risk HPV types.
Vaccinating women over age 26 has not been approved by the U.S. Food and Drug Administration and is not included in U.S. Centers for Disease Control and Prevention guidelines.
An estimated 11,070 new cases of cervical cancer will be diagnosed in 2008 in the United States, and 3,870 women will die from their cancers.
(Editing by Maggie Fox and Vicki Allen)
Sunday, October 26, 2008 | Labels: news | 0 Comments
Colon Cancer Drug Won't Help Those With Certain Gene Mutation
A new study suggests that people with advanced colon cancer who have a particular gene mutation won't benefit from the medication cetuximab (Erbitux).
While the drug can add months to the lives of people without a mutation in a gene called K-ras, those who have the mutation won't see any benefit from this additional therapy, reports the study, which is published in the Oct. 23 issue of the New England Journal of Medicine.
"We believe that, in the context of pre-treated advanced bowel cancer, the K-ras mutation status of the cancer should be determined before using cetuximab, and cetuximab should only be given to patients with tumors that do not have the mutation," said study author Dr. Christos S. Karapetis, a senior consultant medical oncologist and director of clinical research in the department of medical oncology at Flinders Medical Centre in Australia.
Karapetis said that about four in 10 people with colon cancer have the K-ras mutation.
Erbitux works by interrupting cell growth and division. It does this by binding to a receptor known as epidermal growth factor receptor (EGFR). A mutation in the K-ras gene is believed to interfere with cetuximab's ability to disrupt EGFR, according to the study.
For the study, 572 people with advanced colorectal cancer were randomly assigned to receive either weekly treatment with cetuximab and supportive care (287 people) or supportive care alone (285 people). All had undergone other treatment options without success.
Almost 400 tumor specimens from the study volunteers were tested for K-ras mutations (198 from the cetuximab group and 196 from the supportive care group). Just over 42 percent of the tumors evaluated were found to have mutations in the K-ras gene.
Even with cetuximab treatment, people with K-ras mutations had no significant changes in overall survival or in progression-free survival. Those without the mutations, on the other hand, appeared to benefit significantly from the therapy.
People with no K-ras mutations who were treated with cetuximab had nearly twice the overall survival rate compared to the supportive care group -- 9.5 months versus 4.8 months. And, the time of progression-free survival was also nearly doubled for those treated with cetuximab -- 3.7 months versus 1.9 months in the supportive care group.
"Patients with a colorectal tumor bearing mutated K-ras did not benefit from cetuximab," the researchers concluded.
"This study suggests that if someone has this particular mutation, they won't respond to this drug," said Dr. Len Lichtenfeld, deputy chief medical officer for the American Cancer Society. "The bottom line is that this study is important and really has the potential to impact how we treat patients with colorectal cancer with this very expensive drug."
He added that other researchers have noted similar results for K-ras mutations in earlier-stage colorectal cancer.
"This is one more refinement on personalized medicine, and we're moving into an age of molecular markers that eventually will guide treatment. If someone has a cancer in the future, that cancer will be analyzed for what kind of cancer it is, and then we'll know what the best treatments are for that cancer," Lichtenfeld said.
Another important molecular marker that guides treatment is already in use for breast cancer treatment, according to Lichtenfeld. Breast cancers are tested for a type of receptor called HER2. Those with this molecular marker are likely to have a more aggressive type cancer, but also a type of cancer that responds to treatment with the drug trastuzumab (Herceptin), he said.
"I'm excited about the future, and this study shows we can be more targeted with our targeted therapies," said Lichtenfeld.
Sunday, October 26, 2008 | Labels: news | 0 Comments
New Multiple Sclerosis Therapy
THURSDAY, Oct. 23 (HealthDay News) -- Two medications may prove to be advances in the treatment of multiple sclerosis, researchers say.
In one study, an experimental drug called oral fumarate (BG00012) substantially reduced symptoms in patients with relapsing-remitting multiple sclerosis, according to a phase II clinical trial by European and North American researchers.
And in a second trial, researchers found that the leukemia drug alemtuzumab (Campath) was about 70 percent more effective than another drug already widely used to treat MS. However, alemtuzumab also had significant side effects, including bleeding disorders, a greater risk of thyroid disease, and infections. This prompted experts to say that much more research is needed before alemtuzumab can be prescribed to treat multiple sclerosis.
Multiple sclerosis is a nervous system disease that affects the brain and spinal cord. It damages the myelin sheath, the material that surrounds and protects nerve cells. This damage slows or blocks messages between the brain and the body, according to the U.S. National Library of Medicine.
Symptoms of the disease can include visual disturbances; muscle weakness; trouble with coordination and balance; sensations such as numbness, prickling, or "pins and needles;" and thinking and memory problems.
It's not known what causes multiple sclerosis. It may be an autoimmune disease, which happens when the body attacks itself. MS affects women more than men, and it often begins between the ages of 20 and 40. An estimated 400,000 Americans have the disease. Usually, the disease is mild, but some people lose the ability to write, speak or walk. There's no cure for MS, but medicines may slow it down and help control symptoms, according to the National Library of Medicine.
The 24-week study of BG00012 included 257 patients, ages 18 to 55, who were randomly assigned to receive either 120 milligrams of BG00012 once a day (64 patients), 120 milligrams three times a day (64 patients), 240 milligrams three times a day (64 patients), or a placebo (65 patients). The patients were assessed at weeks 12, 16, 20 and 24.
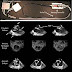
MRI brain scans showed that patients treated with 240 milligrams of BG00012 three times a day had 69 percent fewer new gadolinium enhancing (GdE) lesions -- a marker of MS-related inflammatory activity -- from week 12 to 24 than those who received the placebo. They also had fewer new or enlarging T2-hyperintense and T1-hypointense lesions at week 24.
The study also found that BG00012 reduced the annual relapse rate by 32 percent, but this finding wasn't statistically significant. Patients who received the drug were more likely than those in the placebo group to suffer adverse events such as abdominal pain and hot flush. Dose-related adverse events in patients taking the drug included headache, fatigue and feeling hot, the researchers said.
"Longer-term (phase III) studies of BG00012 in larger patient populations are underway to define its place in the future of relapsing-remitting multiple sclerosis treatment. If these studies show similar relapse rate reductions with BG00012, interferon beta, and glatiramer acetate, BG00012 could be a suitable initial treatment for relapsing-remitting multiple sclerosis," wrote Professor Ludwig Kappos, of University Hospital Basel, in Switzerland, and colleagues.
The study was published in the Oct. 24 issue of the The Lancet.
In an accompanying comment in the journal, Professor Per Soelberg Sorensen and Dr. Finn Sellebjerg of the Danish Multiple Sclerosis Research Center, noted that "BG00012 might have a favorable benefit-to-risk ratio profile compared with its oral competitors and the currently available first-line injectable drugs. However, we will have to await the results from the ongoing large phase III trials to establish the place of BG00012 and of other oral drugs in the treatment of relapsing-remitting multiple sclerosis."
The study of the leukemia drug alemtuzumab, which temporarily depletes white blood cells and is part of a class of drugs called monoclonal antibodies, included 334 patients. Patients were randomly assigned to get either alemtuzumab or interferon beta, a standard MS therapy, for three years.
Alemtuzumab reduced by 74 percent the risk of MS relapse, the researchers reported in the Oct. 23 issue of the New England Journal of Medicine.
"The ability of an MS drug to promote brain repair is unprecedented," Alasdair Coles, of Cambridge University in England, and one of the study's leaders, told the AFP news service. "We are witnessing a drug which, if given early enough, might effectively stop the advancement of the disease and also restore lost function by promoting repair of the damaged brain tissue."
However, in an accompanying journal editorial, Dr. Stephen L. Hauser, a neurologist at the University of California, San Francisco, said the "toxic effects associated with alemtuzumab considerably dampen any enthusiasm for its routine use in patients with multiple sclerosis until more is known about its long-term safety and sustained efficacy."
Sunday, October 26, 2008 | Labels: nervous disorder | 0 Comments